Title: Mutagenesis and structural studies reveal the basis for the specific binding of SARS-CoV-2 SL3 RNA element with human TIA1 protein
Dong Zhang, Lulu Qiao, Xiaobo Lei, Xiaojing Dong, Yunguang Tong, Jianwei Wang, Zhiye Wang & Ruhong Zhou
Abstract
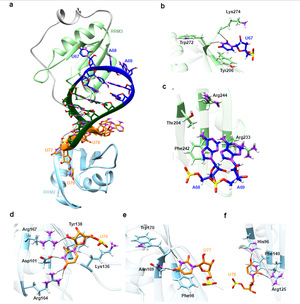
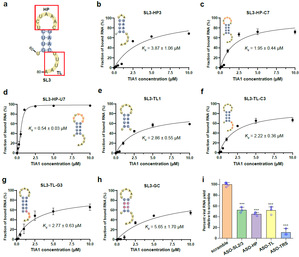
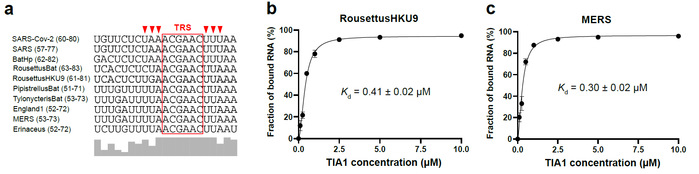
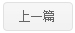
Viral RNA-host protein interactions are indispensable during RNA virus transcription and replication, but their detailed structural and dynamical features remain largely elusive. Here, we characterize the binding interface for the SARS-CoV-2 stem-loop 3 (SL3) cis-acting element to human TIA1 protein with a combined theoretical and experimental approaches. The highly structured SARS-CoV-2 SL3 has a high binding affinity to TIA1 protein, in which the aromatic stacking, hydrogen bonds, and hydrophobic interactions collectively direct this specific binding. Further mutagenesis studies validate our proposed 3D binding model and reveal two SL3 variants have enhanced binding affinities to TIA1. And disruptions of the identified RNA-protein interactions with designed antisense oligonucleotides dramatically reduce SARS-CoV-2 infection in cells. Finally, TIA1 protein could interact with conserved SL3 RNA elements within other betacoronavirus lineages. These findings open an avenue to explore the viral RNA-host protein interactions and provide a pioneering structural basis for RNA-targeting antiviral drug design.
Link: https://www.nature.com/articles/s41467-023-39410-8