Title:The OGT-c-Myc-PDK2 axis rewires the TCA cycle and promotes colorectal tumor growth
Huijuan Wang, Jie Sun, Haofan Sun, Yifei Wang, Bingyi Lin, Liming Wu, Weijie Qin, Qiang Zhu & Wen Yi
Abstract
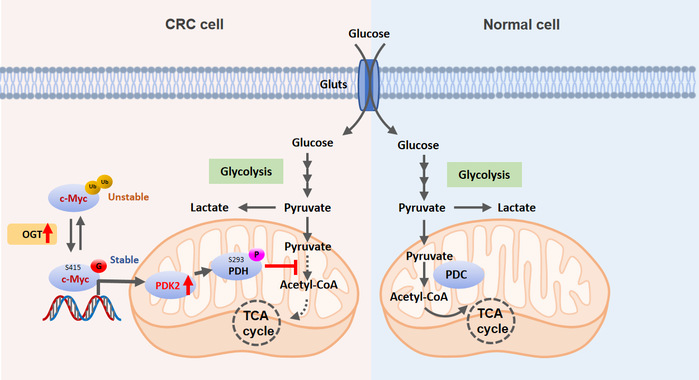
Deregulated glucose metabolism termed the “Warburg effect” is a fundamental feature of cancers, including the colorectal cancer. This is typically characterized with an increased rate of glycolysis, and a concomitant reduced rate of the tricarboxylic acid (TCA) cycle metabolism as compared to the normal cells. How the TCA cycle is manipulated in cancer cells remains unknown. Here, we show that O-linked N-acetylglucosamine (O-GlcNAc) regulates the TCA cycle in colorectal cancer cells. Depletion of OGT, the sole transferase of O-GlcNAc, significantly increases the TCA cycle metabolism in colorectal cancer cells. Mechanistically, OGT-catalyzed O-GlcNAc modification of c-Myc at serine 415 (S415) increases c-Myc stability, which transcriptionally upregulates the expression of pyruvate dehydrogenase kinase 2 (PDK2). PDK2 phosphorylates pyruvate dehydrogenase (PDH) to inhibit the activity of mitochondrial pyruvate dehydrogenase complex, which reduces mitochondrial pyruvate metabolism, suppresses reactive oxygen species production, and promotes xenograft tumor growth. Furthermore, c-Myc S415 glycosylation levels positively correlate with PDK2 expression levels in clinical colorectal tumor tissues. This study highlights the OGT–c-Myc–PDK2 axis as a key mechanism linking oncoprotein activation with deregulated glucose metabolism in colorectal cancer.
Link:https://link.springer.com/article/10.1038/s41418-024-01315-4?utm_source= rct_congratemailt&utm_medium=email&utm_campaign=oa_20240522&utm_content=10.1038%2Fs41418-024-01315-4