Title:cAMP-independent DNA binding of the CRP family protein DdrI from Deinococcus radiodurans
Yudong Wang, Jing Hu, Xufan Gao, Yuchen Cao, Shumai Ye, Cheng Chen, Liangyan Wang, Hong Xu, Miao Guo, Dong Zhang, Ruhong Zhou, Yuejin Hua, Ye Zhao
Abstract
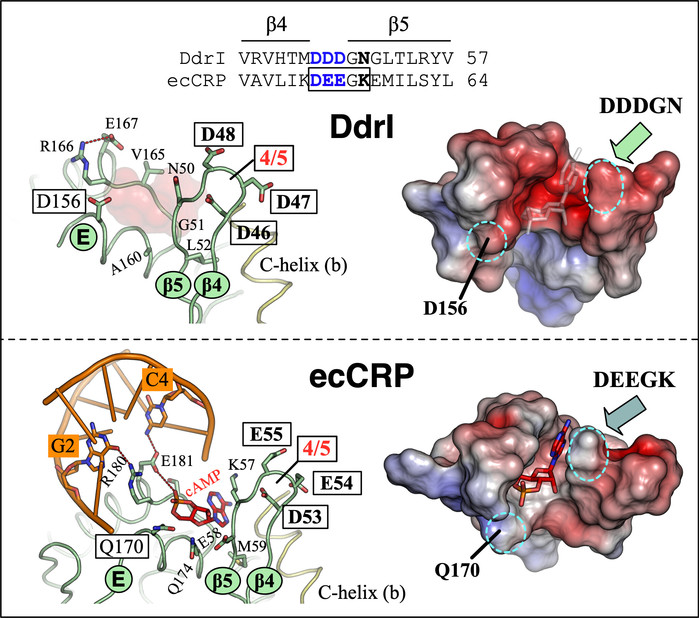
The cAMP receptor proteins (CRPs) play a critical role in bacterial environmental adaptation by regulating global gene expression levels via cAMP binding. Here, we report the structure of DdrI, a CRP family protein from Deinococcus radiodurans. Combined with biochemical, kinetic, and molecular dynamics simulations analyses, our results indicate that DdrI adopts a DNA-binding conformation in the absence of cAMP and can form stable complexes with the target DNA sequence of classical CRPs. Further analysis revealed that the high-affinity cAMP binding pocket of DdrI is partially filled with Tyr113-Arg55-Glu65 sidechains, mimicking the anti-cAMP-mediated allosteric transition. Moreover, the second syn-cAMP binding site of DdrI at the protein-DNA interface is more negatively charged compared to that of classical CRPs, and manganese ions can enhance its DNA binding affinity. DdrI can also bind to a target sequence that mimics another transcription factor, DdrO, suggesting potential cross-talk between these two transcription factors. These findings reveal a class of CRPs that are independent of cAMP activation and provide valuable insights into the environmental adaptation mechanisms of D. radiodurans.
Link: https://journals.asm.org/doi/epub/10.1128/mbio.01144-24